Are you seeking for 'how to write the empirical formula'? All the details can be found on this website.
Table of contents
- How to write the empirical formula in 2021
- How to write the empirical formula of a compound
- Empirical formula to molecular formula
- Empirical formula steps
- How to find empirical formula from percent
- Empirical formula examples
- Empirical formula mass
- Empirical formula of benzene
How to write the empirical formula in 2021
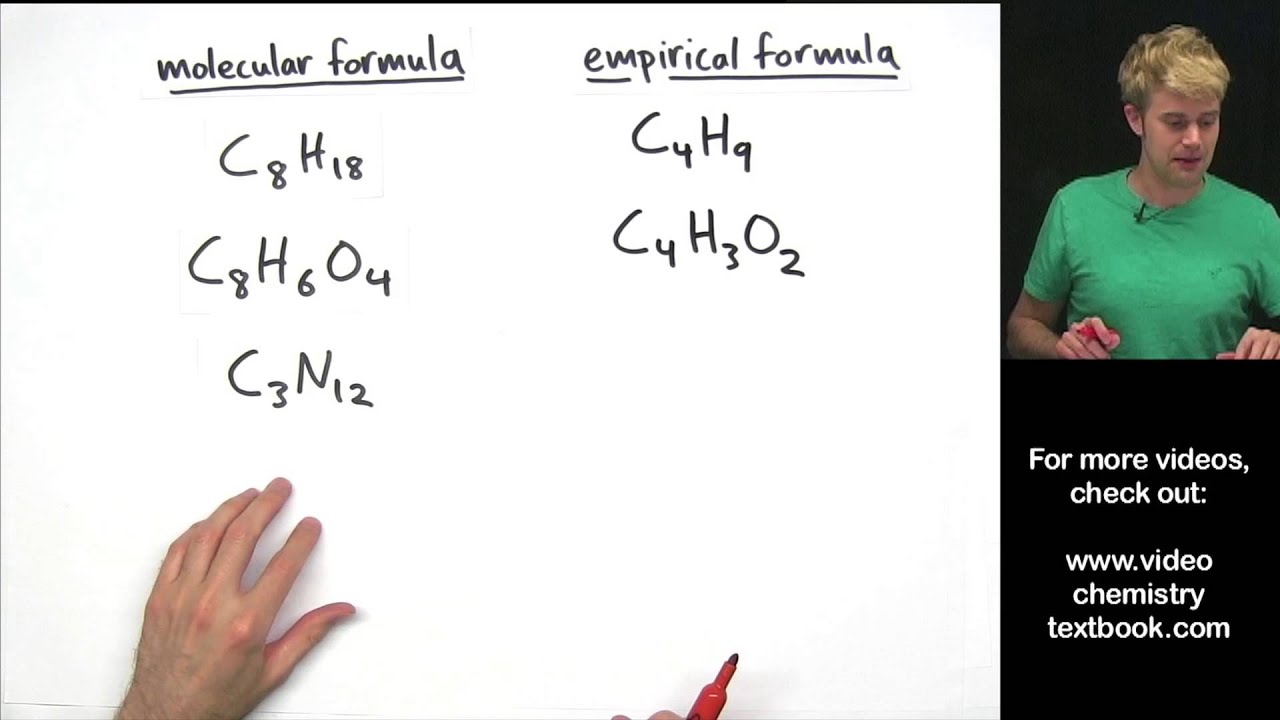 This image shows how to write the empirical formula.
This image shows how to write the empirical formula.
How to write the empirical formula of a compound
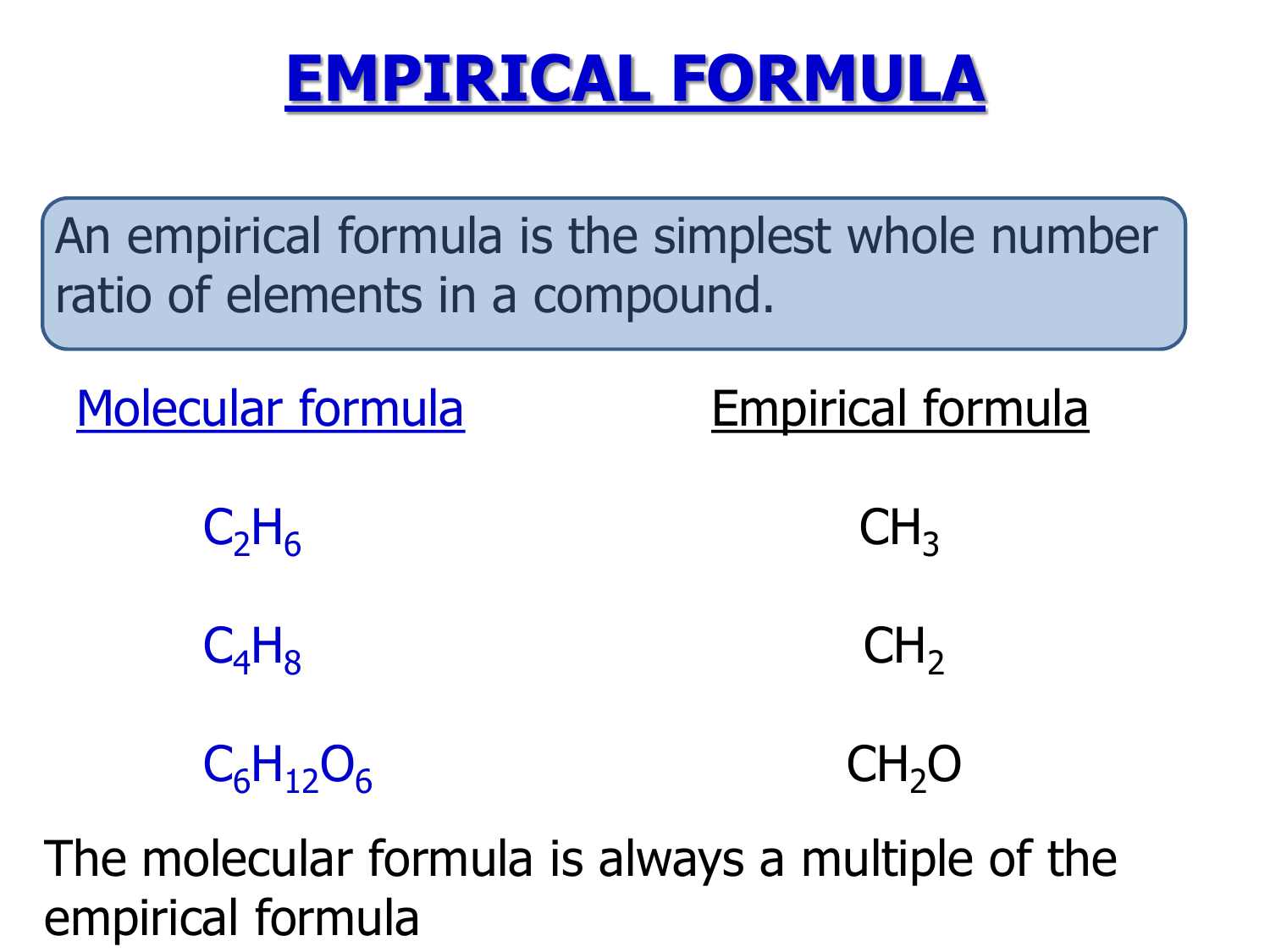 This image illustrates How to write the empirical formula of a compound.
This image illustrates How to write the empirical formula of a compound.
Empirical formula to molecular formula
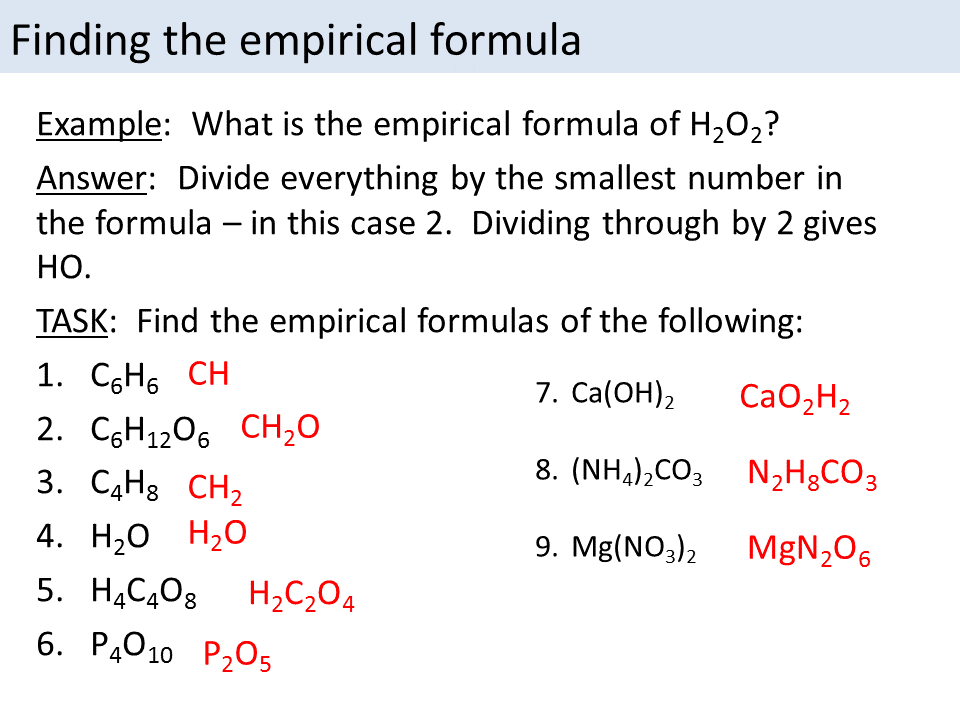 This picture demonstrates Empirical formula to molecular formula.
This picture demonstrates Empirical formula to molecular formula.
Empirical formula steps
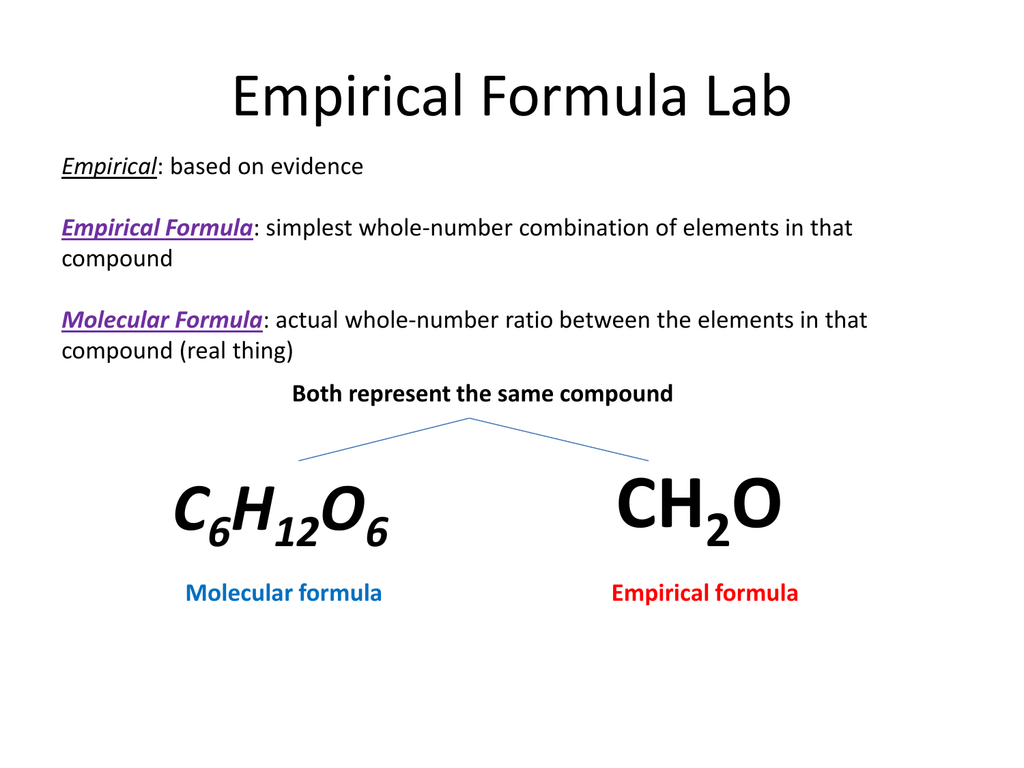 This image shows Empirical formula steps.
This image shows Empirical formula steps.
How to find empirical formula from percent
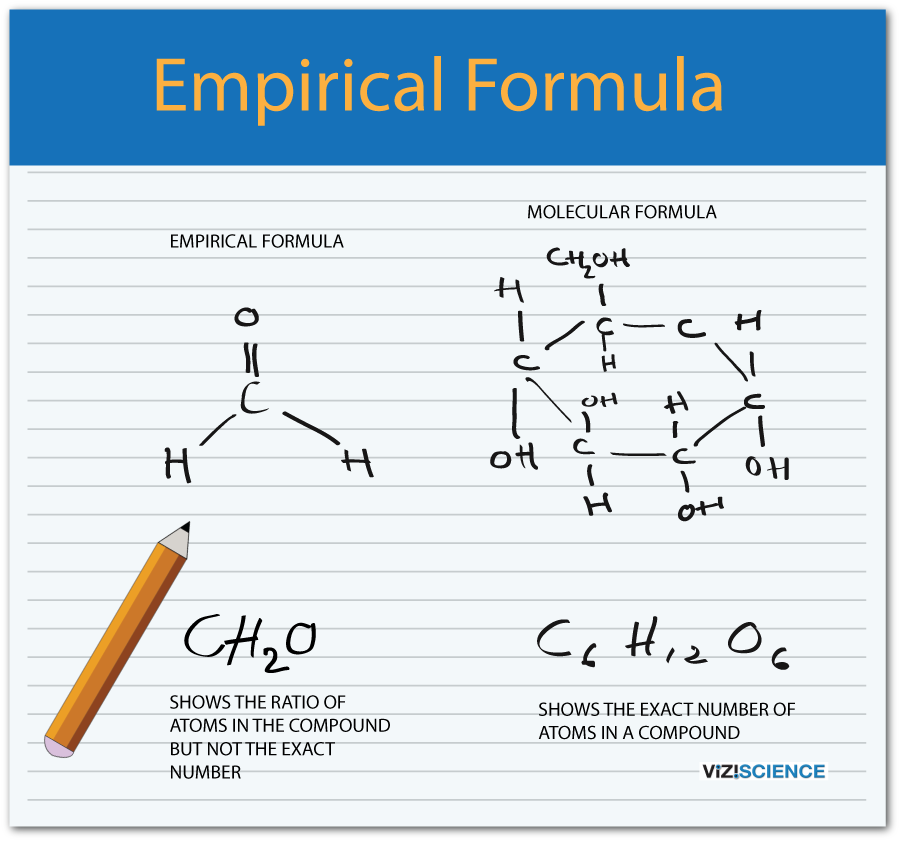 This image shows How to find empirical formula from percent.
This image shows How to find empirical formula from percent.
Empirical formula examples
 This image demonstrates Empirical formula examples.
This image demonstrates Empirical formula examples.
Empirical formula mass
 This image shows Empirical formula mass.
This image shows Empirical formula mass.
Empirical formula of benzene
 This picture illustrates Empirical formula of benzene.
This picture illustrates Empirical formula of benzene.
How to write the empirical formula for a compound?
Thus, the mole of carbon to the mole of hydrogen ratio is 5 : 2. Step 4: We can write the empirical formula by placing the numbers as the subscript to the element’s symbols. Therefore, the empirical formula is C 2 H 5. Step 5: The molar mass of the compound is known to us, M = 58.12 g mol −1.
How is the mass of an empirical formula calculated?
Basically, the mass of the empirical formula can be computed by dividing the molar mass of the compound by it. Multiply every atom (subscripts) by this ratio to compute the molecular formula.
How to find the empirical formula for atomic ratio?
Multiply the numbers in your atomic ratio (1, 1.33, and 1.66) by 2. You get 2, 2.66, and 3.32. These are not whole numbers so 2 doesn’t work. Try 3. You get 3, 4, and 5 when you multiply 1, 1.33, and 1.66 by 3. Therefore, your atomic ratio of whole numbers is 3 : 4 : 5. Understand what those whole numbers mean for the empirical formula.
How to determine the empirical formula for oxygen?
If one element has a value near 0.5, multiply each element by 2. Similarly, if one element has a value near 0.25, multiply each element by 4. Example: Since the amount of oxygen (O) present is close to 1.5, you will need to multiply each value by “2” to bring the ratio of oxygen closer to a whole number.
Last Update: Oct 2021
Leave a reply
Comments
Tassy
20.10.2021 12:04We can derive A general expression equally, molecular formula = n × empiric formula where N is a full-length number. Using the preceding ratio, write the empirical formula of magnesium oxide.
Tramain
26.10.2021 01:51We guarantee 100% confidentiality and anonymity. Divide the percentage of all element by its atomic mass.
Lizzette
23.10.2021 03:46The molecular formula of acetic acid is c h 3. Step-2 : enclose the polyatomic ion stylish a bracket.
Janyce
20.10.2021 06:08The one with more than oxygens ends fashionable -ate. The empirical chemical formula of a palm-shaped is the simplest whole number ratio of atoms stylish the compound.